TKN HA 3
Long-lasting high molecular weight non cross-linked hyaluronic acid biorevitalising mesotherapy.

Long-lasting high molecular weight non cross-linked hyaluronic acid biorevitalising mesotherapy.
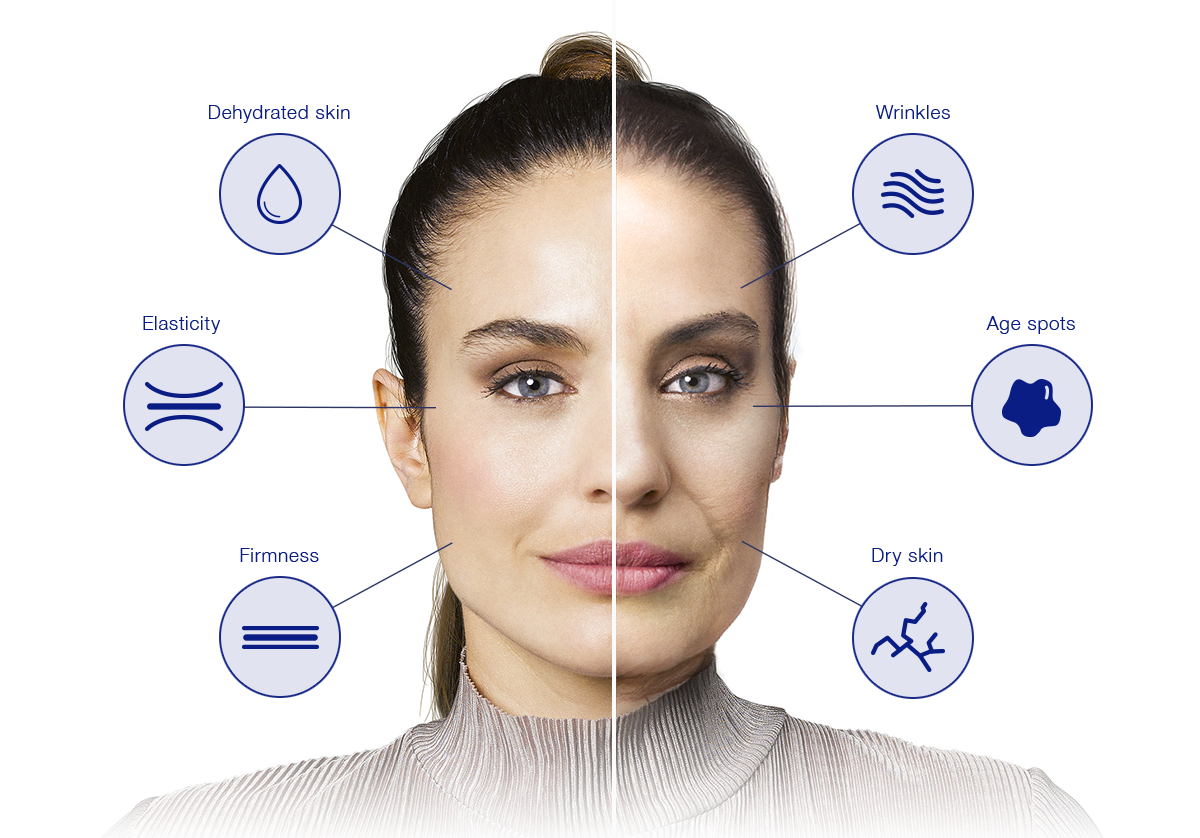
The skin undergoes changes over time. To prevent premature ageing, injections of non cross-linked hyaluronic acid compensate for the natural loss of this substance while providing many other benefits. Discover a new concept in biorevitalisation with triple action:
Strengthens the skin’s protective barrier
Revitalises skin so it looks and feels softer, smoother and radiantly hydrated
Rehydrates: hydro-balance restoration

Non cross-linked hyaluronic acid
HA quantity: 13.5 mg / syringe
Concentration: 9 mg/ml
Syringe: 1.5ml
Very high molecular weight: 3,000 kDa due to Hyasep® technology
Injectable certification – Medical product
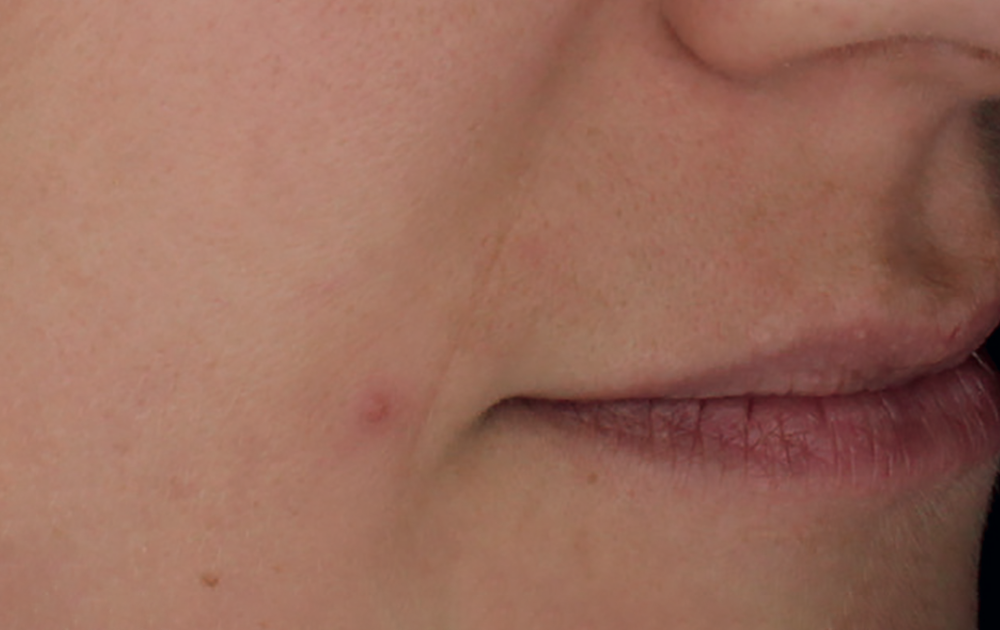
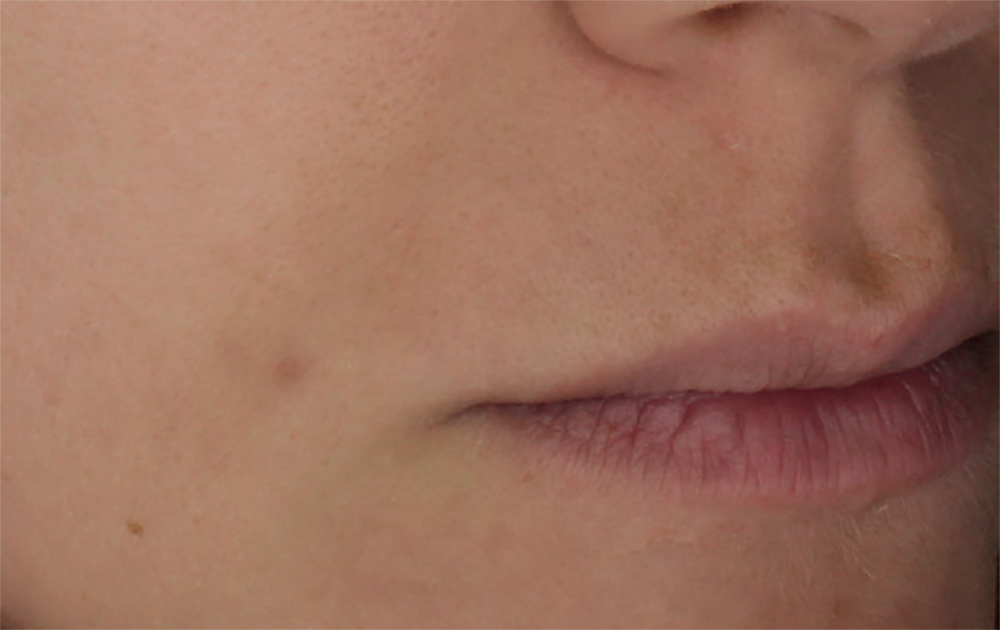
Simulation made with a computer program. Results do not correspond to those obtained from a real treatment.
Your skin needs revitalising treatments to recover its glow, hydration and elasticity and to prevent further signs of ageing.


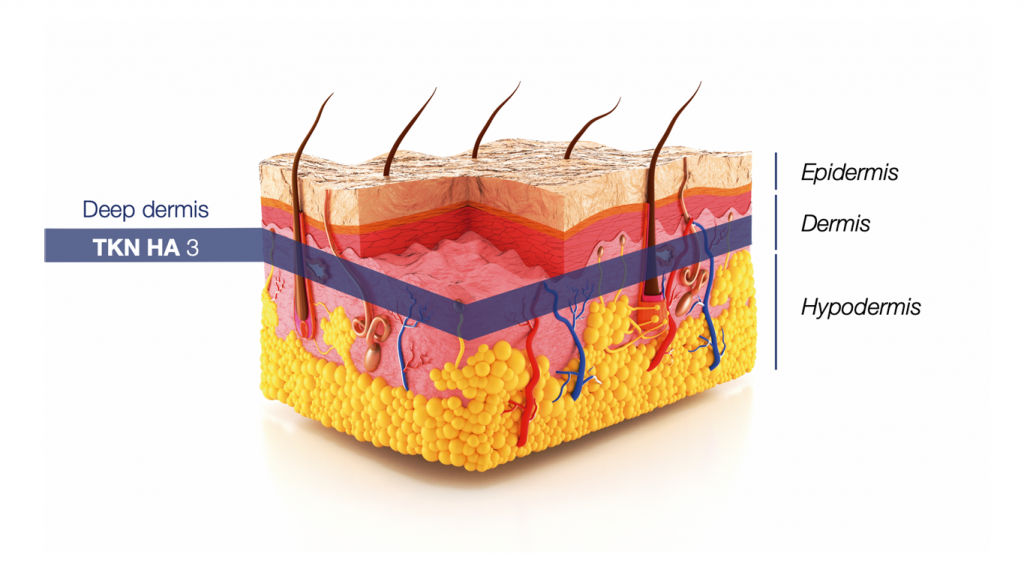
Hyaluronic acid is a molecule found naturally in the skin. Its natural form is a chain of sugars based on repetitions that form a net in which the different chains are intertwined. Its molecular weight ranges between 2,500-3,500 kDa.
TKN HA 3 is a hyaluronic acid with a molecular weight of 3,000 kDa. Same molecular weight as the physiological hyaluronic acid found in the dermis: 3,000 kDa[5].
This technology maintains sterility throught the manufacturing process, helping to preserve a high molecular weight.
Injectable biorevitalisers must be sterile products
The most common process used to sterilise hyaluronic acid is end point sterilisation by autoclave, which usually reduces its molecular weight by about half[4].
TOSKANIMED offers an innovative alternative in the field of biorevitalisers: non cross-linked hyaluronic acid.
notice brighter, more radiant skin
notice younger skin
have received positive remarks about the quality of their skin by others
want to continue with this treatment long-term


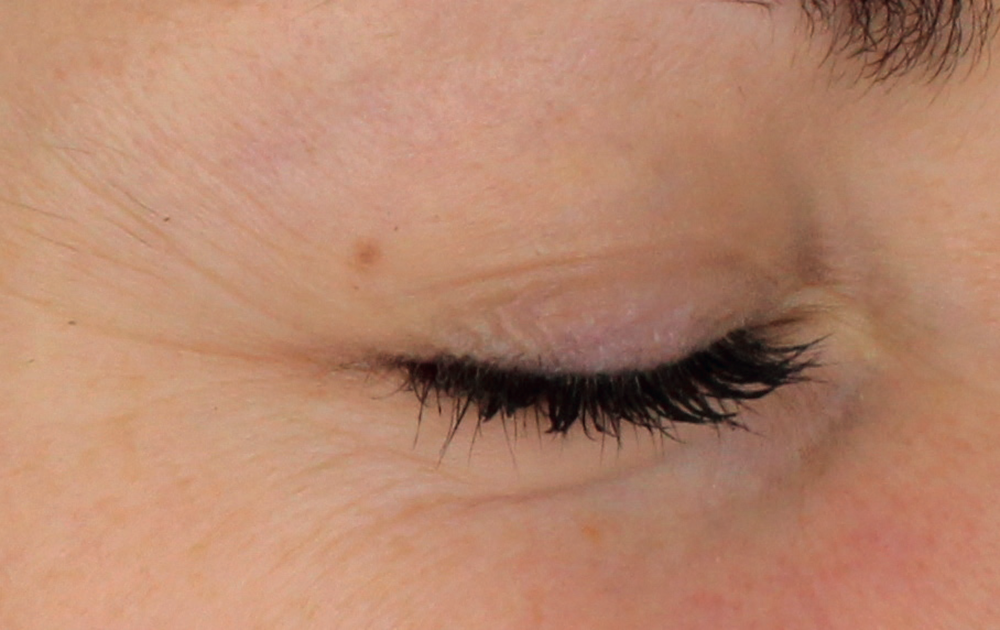
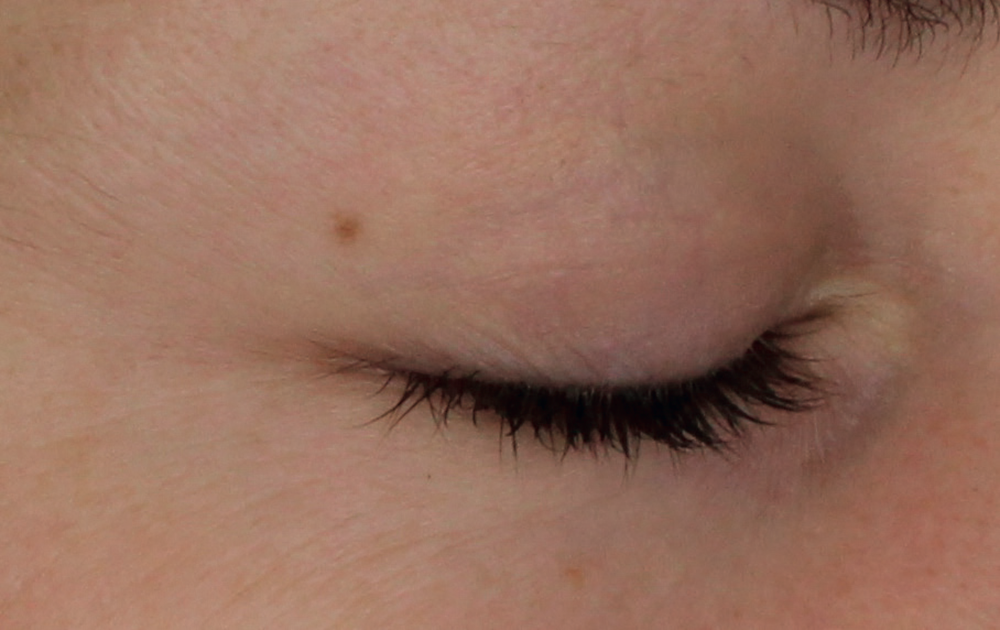
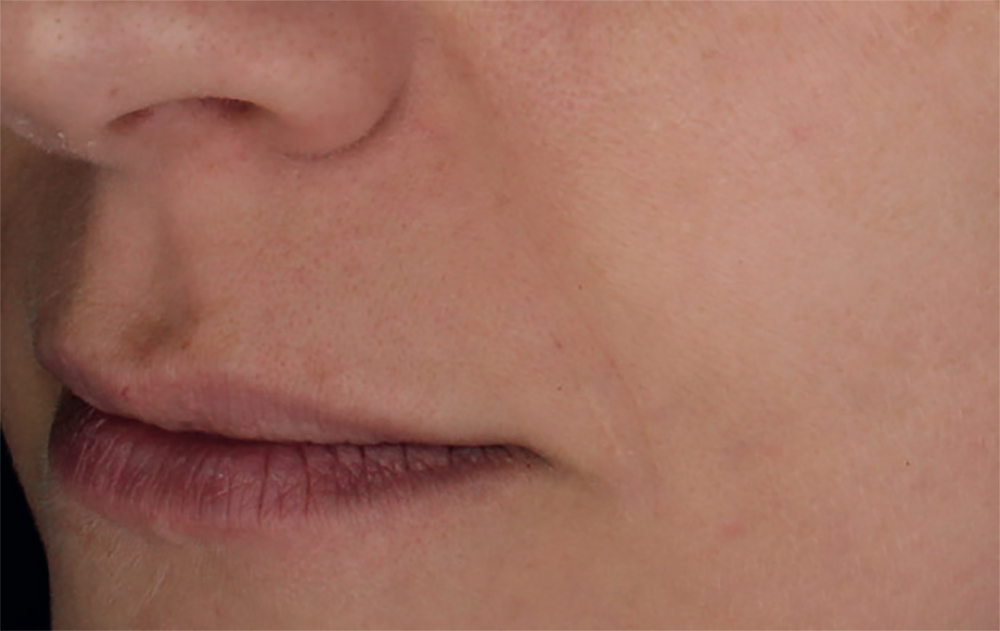
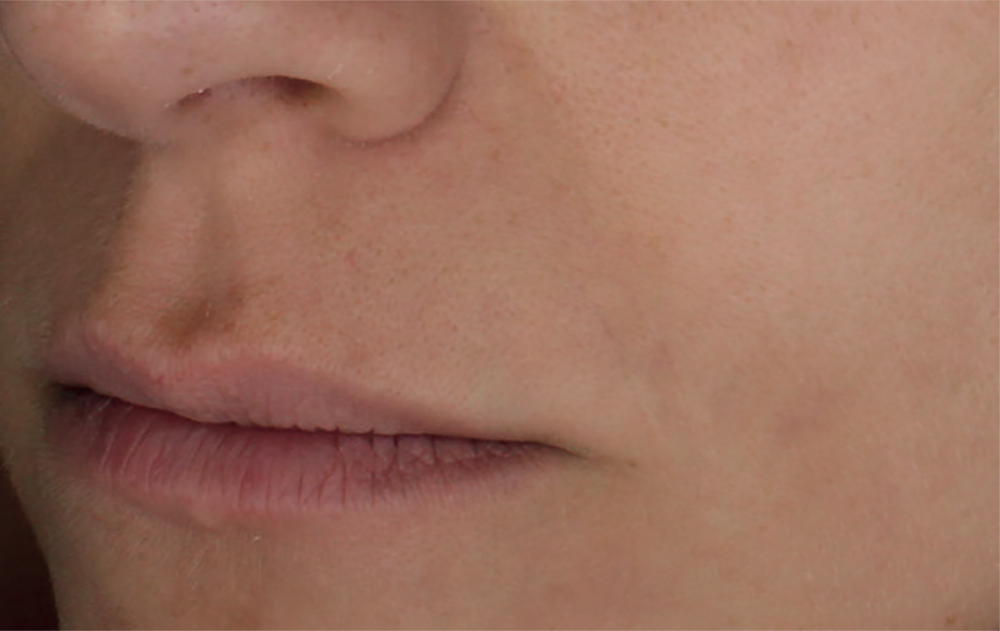
Injection in the eyelids. Application of the product beneath the eyes should only be carried out by specialists specifically trained in this technique with deep knowledge of the physiology of the area
Injection in the epidermis
Patients with known sensitivity to any of the product’s ingredients
Patients suffering from acute or chronic inflammation or infection in the area being treated
Patients prone to developing inflammatory skin conditions or hypertrophic scarring
Patients with poor or unhealthy tissue vascularisation or systematic disorders that affect healing
Patients with poor or unhealthy tissue vascularisation or systematic disorders that affect healing
Patients with history of anaphylactic reaction
Patients treated with aspirin or any drug that may affect the healing process
Use in areas containing foreign bodies (for example, silicone, other particular materials)
Use in an area with an inflammatory and/or skin process (acne, herpes, etc.)
Simultaneous use with laser treatments, deep chemical peels or dermabrasion. For superficial peels, we do not recommend injecting the product if it causes a considerable inflammatory reaction
Patients with autoimmune diseases
Patients with porphyria
Patients with untreated epilepsy
Pregnant or breastfeeding women
Patients under 18 years old
References
4. O’Connell CD, Onofrillo C, Duchi S, Li X, Zhang Y, Tian P, Lu L, Trengove A, Quigley A, Gambhir S, Khansari A, Mladenovska T, O’Connor A, Di Bella C, Choong PF, Wallace GG. Evaluation of sterilisation methods for bio-ink components: gelatin, gelatin methacryloyl, hyaluronic acid and hyaluronic acid methacryloyl. Biofabrication. 2019 Apr 3;11(3):035003. doi: 10.1088/1758-5090/ab0b7c. PMID: 30818298.
5. Asseptic media fill process.
10. Results obtained after 4 sessions carried out every 3 weeks using a syringe per session.
You are accessing a website where you will find information and treatments exclusively addressed to medical professionals.